Abstract
Background: Myelodysplastic syndrome (MDS) is a clinically heterogenous disease of hematopoietic stem cells (HSC) characterized by ineffective hematopoiesis, uni/multi-lineage dysplasia and a high tendency to transform into acute myeloid leukemia. Aberrant chromosomal and genetic lesions contribute to MDS pathogenesis which has been associated with chronic activation of the innate immune response and a hyperinflammatory microenvironment (Barryero L, et al. Blood, 2018). Dysfunction of Toll like receptors (TLR) and downstream effectors has been associated with the loss of progenitor function and differentiation of bone marrow (BM) cells in MDS patients. Azacitidine (AZA), a hypomethylating agent (HMA), is the mainstay of therapy for patients with higher-risk MDS (Silverman LR, The Myelodysplastic Syndrome in Cancer Medicine, Editors: R.J. Bast, et al. 2017) and carries an overall response rate (ORR) of 50% in patients with significant effects on hematopoiesis, ranging from improvement in a single lineage to complete restoration of blood counts and transfusion independence with survival benefits (Silverman LR, et al., Leukemia, 1993). The response to AZA is not durable and all patients relapse with worsening bone marrow failure.
The paradigm of MDS therapy is now shifting to combinatorial drug treatment to overcome single-agent HMA resistance in higher-risk MDS patients. Rigosertib (RIGO), a Ras mimetic which had been shown to interfere with the Ras-Raf binding domain, has limited single-agent activity (ORR 15%) and failed to provide a survival benefit compared to standard of care in MDS patients failing an HMA. RIGO combined with AZA produced an ORR of 90% in HMA naïve patients and 54% in patients who failed HMA (Navada SC, et al. EHA Library 2019). This represents a critical observation in overcoming the epigenetic clinical resistance phenotype. The mechanism is still unclear.
Method: We therefore investigated the pathways that are perturbed by AZA and RIGO monotherapy and in combination (RIGO-AZA). We used the MDS-L cell line as a model to limit the heterogeneity observed in MDS patients. The cells were treated with RIGO, AZA, and RIGO-AZA for 48 hrs and further analyzed by qPCR and western blot.
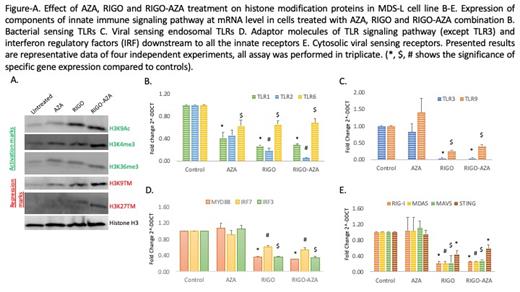
Result: We found an increase in H3K9ac protein expression with RIGO and RIGO-AZA; AZA and RIGO alone each had similar effects on H3K4me3, however, its expression was markedly upregulated with RIGO-AZA (Figure A). Effects on H3K36me3 were comparable in all treated cells. We observed marked effects on the repression marks H3K9me3 and H3K27me3 by RIGO and RIGO-AZA combination (Figure A). Furthermore, we studied the expression of bacterial sensing TLRs (1, 2 and 6), viral sensing endosomal TLRs (3 and 9), and cytosolic viral particle sensing receptors like Retinoic acid inducible gene (RIG)-I, Melanoma differentiation-associated protein 5 (MDA5) and Stimulator of interferon genes (STING); their intermediate adaptor molecules Myeloid differentiation factor 88 (MYD88) (for all TLRs except TLR3), mitochondrial antiviral signaling (MAVS) gene (for RIG-I and MDA5); and interferon regulatory factor (IRF)-3 and -7 by qPCR. We observed that AZA, RIGO, and RIGO-AZA significantly inhibit the expression of TLR1, 2 and 6 (Figure B). However, TLR-3, 9, RIG-I, MDA5, STING, MAVS, MYD88, and IRF-3, 7 were significantly inhibited by RIGO and RIGO-AZA (Figure C-E).
Conclusion: RIGO has effects on innate immune signaling and histone modification of both activator and repressor marks. Further studies are underway to determine the correlation of the histone modification and innate immune signaling changes, and if these mechanisms contribute to the improvement in hematopoiesis in MDS patients.
Navada: Janssen Pharmaceuticals, Inc.: Current Employment. Reddy: Onconova Therapeutics, Inc.: Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal